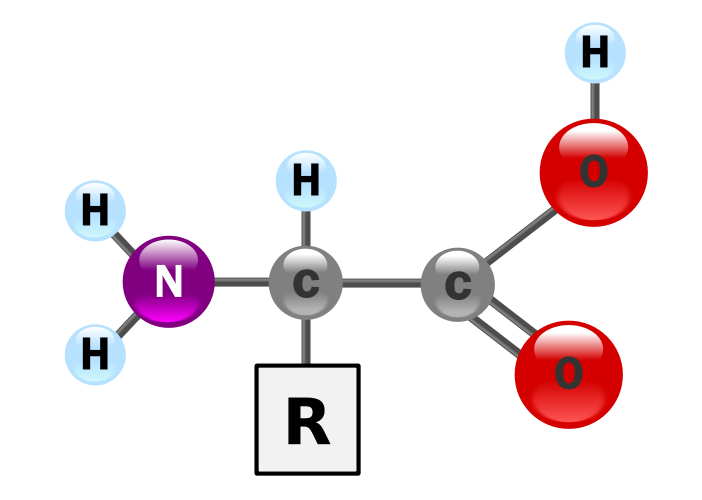
 Last week I introduced the idea of using two dyes to illustrate the power of ion exchange chromatography (IEC). The dyes were used instead of amino acids, but served to illustrate how the net charge on a molecule can be used as a means of purifying (preparative ion exchange chromatography) or simply analysing mixtures of molecules, such as amino acids (LHS). Consider the 4 of the amino acids found in proteins: Glutamate (Glu), Aspartate (Asp), Tyrosine (Tyr) and Lysine (Lys). In addition to their shared amino and carboxylate groups (which in large part define their properties as free amino acids), Glu and Asp have a second carboxylate group as the side chain. Thus at neutral pH, they carry a net negative charge. By comparison, Lys is positive, owing to an additional amino group, whilst Tyr is polar, but neutral, owing to the hydroxyl group attached to its aromatic side chain. Look the structures up at the wikipedia site and satisfy yourselves that you appreciate the chemical properties of each amino acid. Forthe purposes of the lab sessions I will focus on IEC, since gel filtration chromatography (sometimes called gel permeation), elegant though it is, has limited application and affinity chromatography is specific (by its very nature) to single classes of proteins. You will also have last year's experimental report on the use of Ni-NTA Affinity chromatography for the purification of tagged GFP. See my Blog here. The use of solvents and silica based resins in thin layer chromatography and high performance liquid chromatography, are discussed below.
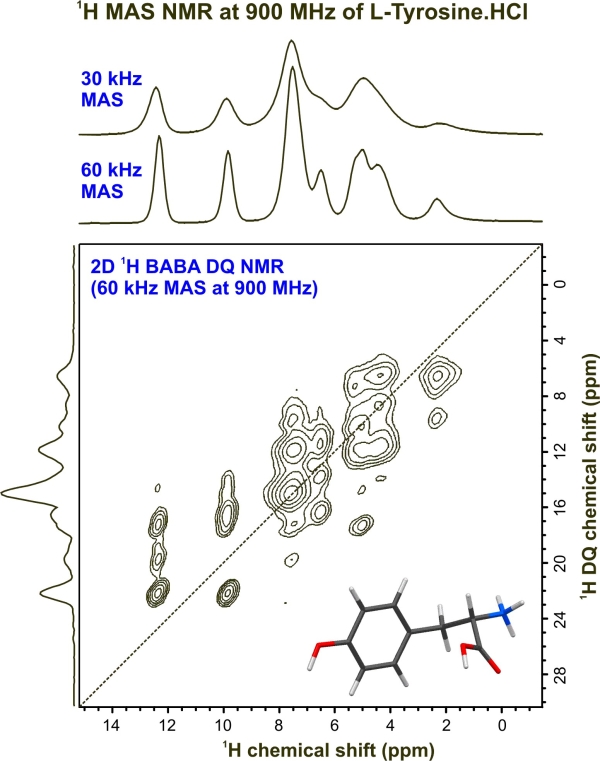
Last week I introduced the idea of using two dyes to illustrate the power of ion exchange chromatography (IEC). The dyes were used instead of amino acids, but served to illustrate how the net charge on a molecule can be used as a means of purifying (preparative ion exchange chromatography) or simply analysing mixtures of molecules, such as amino acids (LHS). Consider the 4 of the amino acids found in proteins: Glutamate (Glu), Aspartate (Asp), Tyrosine (Tyr) and Lysine (Lys). In addition to their shared amino and carboxylate groups (which in large part define their properties as free amino acids), Glu and Asp have a second carboxylate group as the side chain. Thus at neutral pH, they carry a net negative charge. By comparison, Lys is positive, owing to an additional amino group, whilst Tyr is polar, but neutral, owing to the hydroxyl group attached to its aromatic side chain. Look the structures up at the wikipedia site and satisfy yourselves that you appreciate the chemical properties of each amino acid. Forthe purposes of the lab sessions I will focus on IEC, since gel filtration chromatography (sometimes called gel permeation), elegant though it is, has limited application and affinity chromatography is specific (by its very nature) to single classes of proteins. You will also have last year's experimental report on the use of Ni-NTA Affinity chromatography for the purification of tagged GFP. See my Blog here. The use of solvents and silica based resins in thin layer chromatography and high performance liquid chromatography, are discussed below. Separation of the four molecular species discussed above, presents a real challenge, but one that has been solved by applying a range of chromatography methods. A second important part of any separation method is detection. Glu and Asp differ from each other only by an a single methylene (CH2) group, but from the other two by possessing 2 carboxylate groups. Similarly, Lys differs from the others since it has two amino groups and finally Tyr has a polar aromatic side chain. Let us say that we had 4 separate samples of the 4 amino acids. How could we distinguish between them, rapidly and economically? There are several methods that can distinguish each of the amino acids, but these are not widely available in a typical Life Sciences Laboratory. Mass spectrometry will provide a clear answer, since it is possibly to distinguish between molecules differing by 1Da. Infrared spectroscopy measures differences in the vibrational characteristics of intramolecular bonding and NMR (above right) will readily distinguish between the four amino acids on the basis of differences in absorption characteristics of the protons in an applied magnetic field. One final point is that Tyr will absorb in the ultra violet region, since it has an aromatic ring as a side chain.

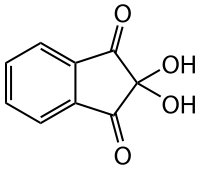 All of the above physical methods are not always available and sometimes samples may be available in too low a yield to obtain definitive results. One of the early methods employed for the analysis of amino acids was to combine paper chromatography, or later thin layer chromatography (TLC) with a range of solvents and to use the molecule ninhydrin, to provide a sensitive indication of the presence of an amino acid. Ninhydrin (top LHS) reacts with primary (and secondary) amines to give a purple colour (I think secondary amines are yellow). If all 4 amino acids are applied to a TLC plate and the plate dipped into a solvent reservoir and allowed to "develop", the amino acids can be separated owing to their differential "retention" by the stationary phase used for the separation (usually silica) on the TLC plate. The result of a typical separation by TLC is shown top right, for a mixture of dye molecules. The term TLC is used for a range of different resins and you can find a detailed discussion here. For the details of the separation of amino acids, follow this link. Briefly, separation can be achieved owing to the difference in the affinity of each amino acid for the silica and its relative solubility in the solvent. As you might imagine, it is easy to separate Tyr from Lys or Glu, but not Glu from Asp.
All of the above physical methods are not always available and sometimes samples may be available in too low a yield to obtain definitive results. One of the early methods employed for the analysis of amino acids was to combine paper chromatography, or later thin layer chromatography (TLC) with a range of solvents and to use the molecule ninhydrin, to provide a sensitive indication of the presence of an amino acid. Ninhydrin (top LHS) reacts with primary (and secondary) amines to give a purple colour (I think secondary amines are yellow). If all 4 amino acids are applied to a TLC plate and the plate dipped into a solvent reservoir and allowed to "develop", the amino acids can be separated owing to their differential "retention" by the stationary phase used for the separation (usually silica) on the TLC plate. The result of a typical separation by TLC is shown top right, for a mixture of dye molecules. The term TLC is used for a range of different resins and you can find a detailed discussion here. For the details of the separation of amino acids, follow this link. Briefly, separation can be achieved owing to the difference in the affinity of each amino acid for the silica and its relative solubility in the solvent. As you might imagine, it is easy to separate Tyr from Lys or Glu, but not Glu from Asp.  On a separate note, because Lys side chains are commonly found on the surface of proteins, ninhydrin can be used in a spray to detect fingerprints. If a person presses their finger onto a surface, small amounts of protein are transferred from the dead skin. By reacting the surface with ninhydrin, otherwise invisible fingerprints are revealed in forensic analysis, as shown on the left. It should also be pointed out that ninhydrin (which is usually dissolved in acetone) is hazardous and must be used in a fume hood, with appropriate safety precautions (eg gloves, mask etc).
On a separate note, because Lys side chains are commonly found on the surface of proteins, ninhydrin can be used in a spray to detect fingerprints. If a person presses their finger onto a surface, small amounts of protein are transferred from the dead skin. By reacting the surface with ninhydrin, otherwise invisible fingerprints are revealed in forensic analysis, as shown on the left. It should also be pointed out that ninhydrin (which is usually dissolved in acetone) is hazardous and must be used in a fume hood, with appropriate safety precautions (eg gloves, mask etc).The most common method used for the separation of all 20 amino acids (well, 19 since technically Pro is an imino acid) found in proteins, is High Performance Liquid Chromatography (HPLC). We do have such an instrument in the laboratory, but the separation and detection of amino acids requires a specialised chromatography column and definitive detection requires the use of NMR (or one of the other physical methods). Nevertheless, HPLC is a powerful method which employs the principles of TLC in a cylindrical column, with sophisticated pumping systems which can deliver solvent mixtures precisely in order to separate small molecules on the basis of subtle differences in their affinity for the column resin and the solvents applied. See here for a detailed description.
Returning to the lab experiment, you should look up the structures of brilliant blue and safranin dyes, and decide which groups of amino acids share their net charge. Given that the column resin has a net positive charge, which dye should behave like Glu and Asp and which one like Lys? Secondly you should comment on whether the addition of 1M sodium chloride led to the displacement of the dye or dyes and explain your results. When I looked around the lab, everyone seemed to have obtained the expected results, but you must not only report and explain your findings, but you should comment on the choice of methods for separating amino acids and the limitations of the methods available with respect to the small differences in the chemistries of the amino acids. The importance of the definitive nature of the methods of detection should also be covered in your discussion. Finally, chromatography represents an excellent example of the predictability of scientific methodology. The sensitivity of the best methods of chromatography, to subtle differences in the chemical and physical properties of molecules, make it on the one hand a powerful methodology, but on the other places considerable demand on the operator in respect of attention to detail during an experiment!